When two non-metal elements combine they form a binary molecular compound. View a sample solution.

Chemistry 101 Naming Binary Molecular Compounds Youtube
Binary acidsconsist of two elements usually hydrogen and one of the halogens.
. For example the compound NaOH is called sodium hydroxide because it contains the Na sodium cation and the OH hydroxide anion. P 4 S 10. This article covers the information related to the rules for naming binary molecular compounds.
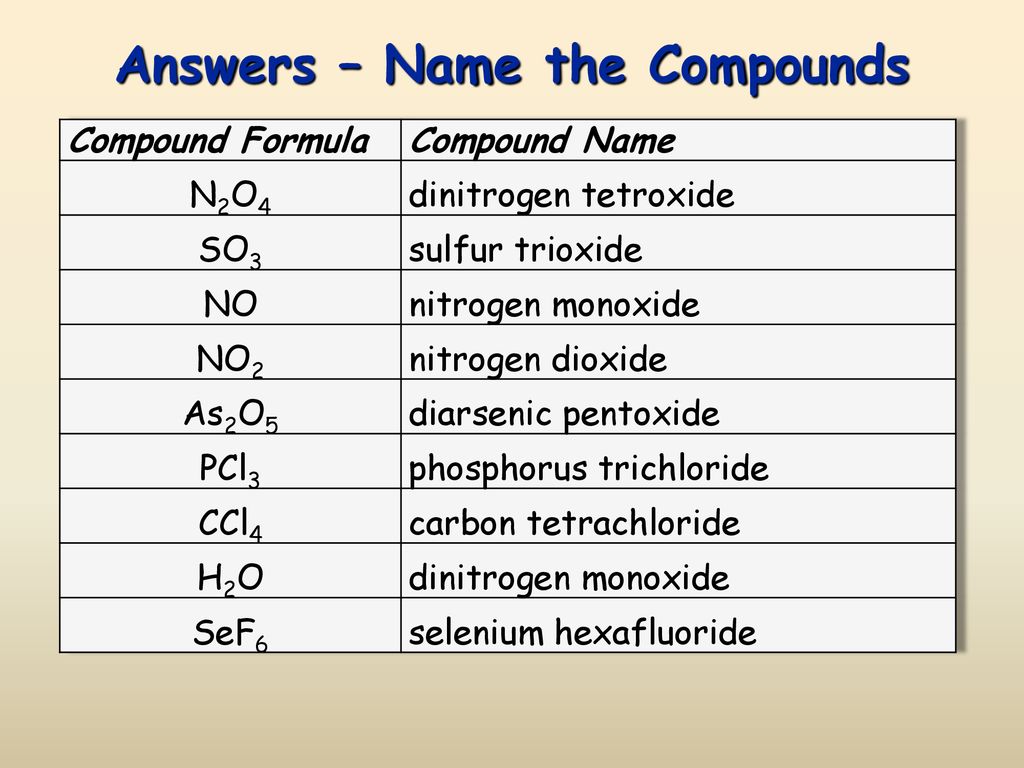
Name the following binary molecular compounds. For example hydrochloric acid refers to compound hydrogen chloride HCl dissolved in water. S 2 F 10.
B F 3. If just one atom of the first element is in the. Here is a guide to writing formulas from binary molecular compounds.
100 3 ratings for this solution. The nonmetal elements are. A calcium oxide CaO.
Write the names of the elements in the order listed in the formula. BiNO33 diegoalacayo diegoalacayo 02222017 Chemistry High School answered What is the formula of a binary compound. View this answer View this answer View this answer done loading.
3 aq is a. The nomenclature for Binary molecular compounds is described in the following set of rules. 3 Write the suffix -ic if the polyatomic ion ends in -ate or -ous if the polyatomic ion ends in.
2 Write the base name of the polyatomic ion. Write the formula for each binary covalent compound. 1 Identify the polyatomic ion in the acid formula.
Most acids can be classified as one of two types. As in binary ionic compounds when a metal that can form multiple cations is present a Roman numeral is. Join our Discord to connect with other students 247 any time night or day.
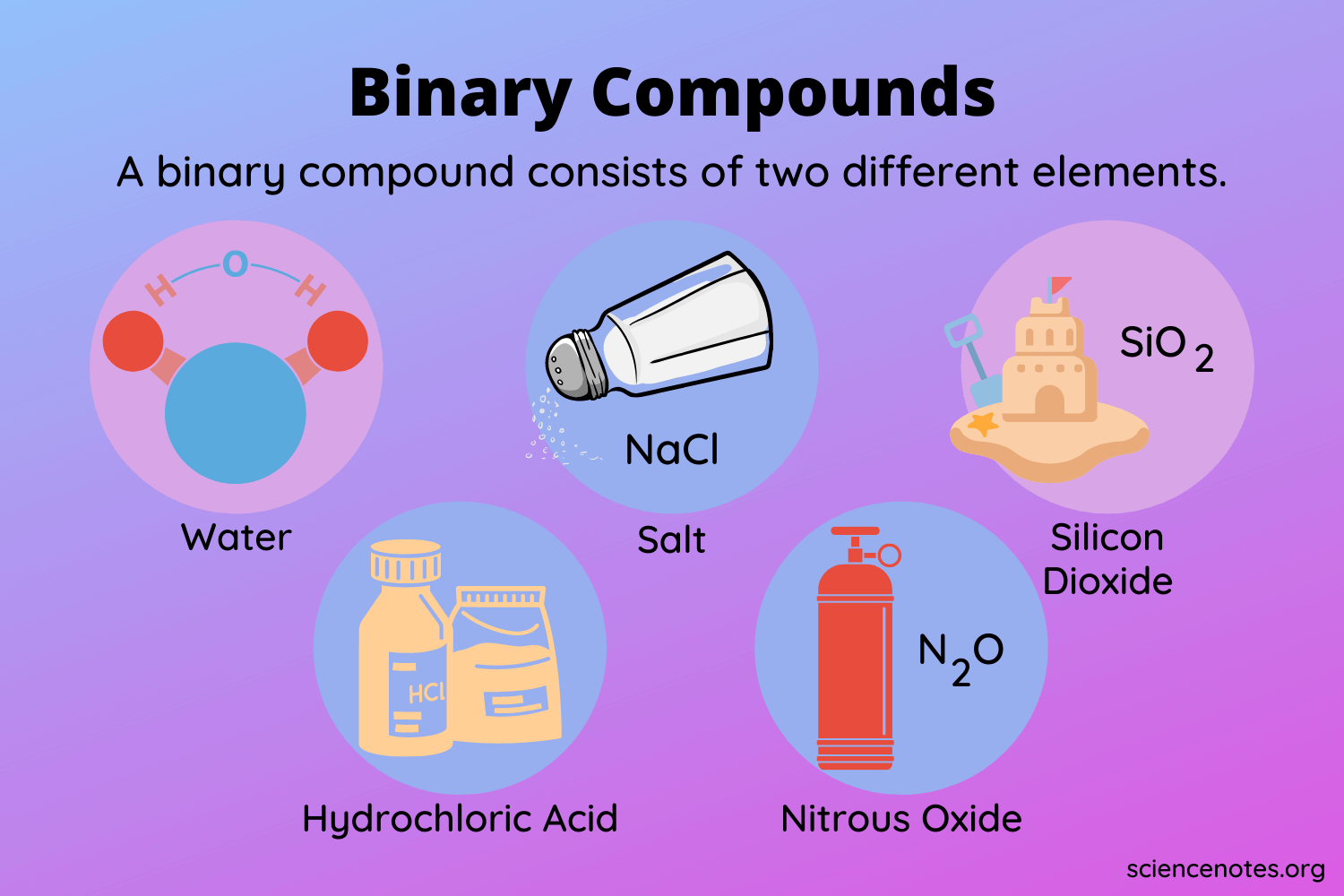
A binary molecular compound is a compound that is form by 2 nonmetal elements. These compounds combine by forming a covalent bond between the non-metallic elements. Classify each of the following as a binary ionic compound ternary ionic compound binary molecular compound binary acid or ternary oxyacid.
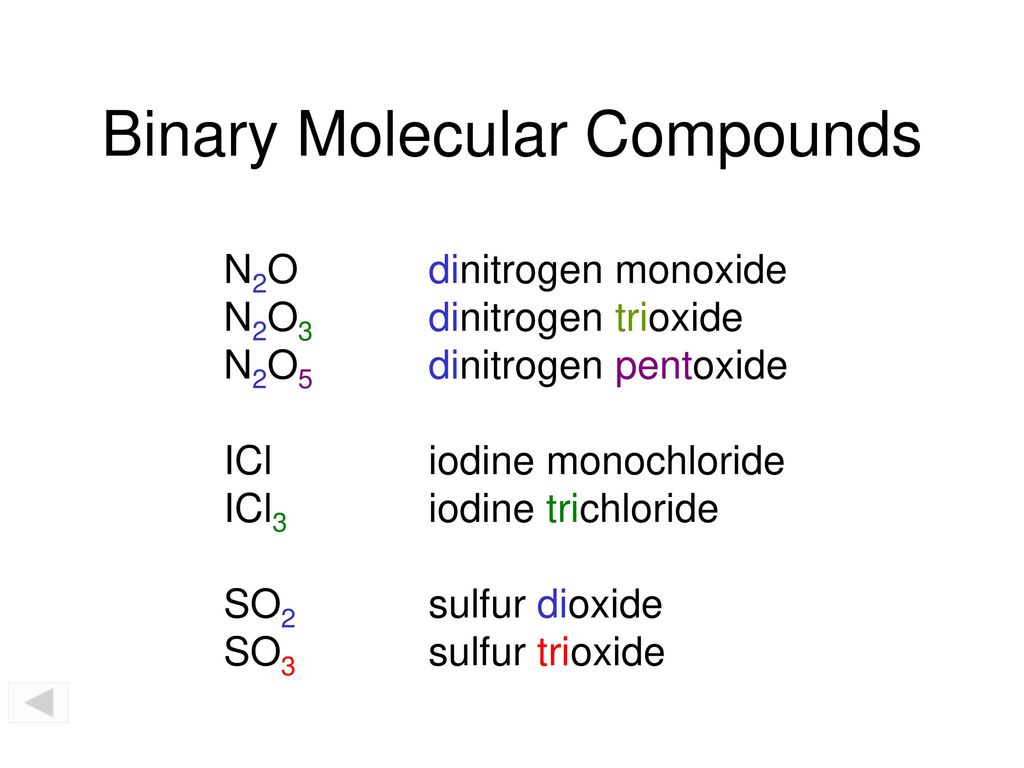
Prefix Name of first non-metal Prefix Name of second non-metal ide. The more positive atom is written first the atom which is the furthest to the left and to the bottom of the periodic tableThe more negative second atom has an -ide ending. And in naming molecular compounds you need to use numerical prefixes to indicate how many of each type of many atoms of each element you have Were always here.
Binary Ionic Compounds Binary Covalent Compound Nonbinary Compound. Some of the common examples include H 2 O NaCl MgCl CO etc. Step 1 of 5.
B CNOF SIP SCl As Se Br Te L At and H. To name a binary molecular compound use the following guidelines. Step 3 of 5.
Which of the following isare covalent binary compounds. 3 aq is a compound containing three elements including hydrogen and oxygen dissolved in water. N 2 O 4.
An oxygen molecule consists of two oxygen atoms whose total mass is 53 x 10-26 kg and whose moment of inertia about an axis perpendicular to the line joining the two atoms midway between them is 182 10-46 kgm2. Write the chemical symbol for the first of the two elements named. As they do not form ionic bonds it.
Determine the subscript needed on the first. Step 2 of 5. Write your answer in order with the first at the top and the last at the bottom.
33 rows A B. On the other hand CO2 is composed of oxygen and carbon atoms that are covalently bound to. Sodium chloride and potassium hydroxide are certainly binary containing only TWO or THREE types of atoms but they are NOT molecular and consist of infinite arrays of positively and negatively charged ions the which are held together by electrostatic interaction.
Step 4 of 5. BiNO33 1 See answer Advertisement. Which of the following is a binary molecular compound A.
Oxyacidscontain hydrogen oxygen and a third element usually a nonmetal. Use prefixes appropriately to indicate the number of each kind of atom. They are located on the right side of the periodic table with the exception of H that is on the top left because it shares properties with the elements that are on that side of the periodic table.
Chapter 5 Problem 7PE is solved.
Binary Molecular Compounds Naming And Formulas Study Guide Inspirit

0 Comments